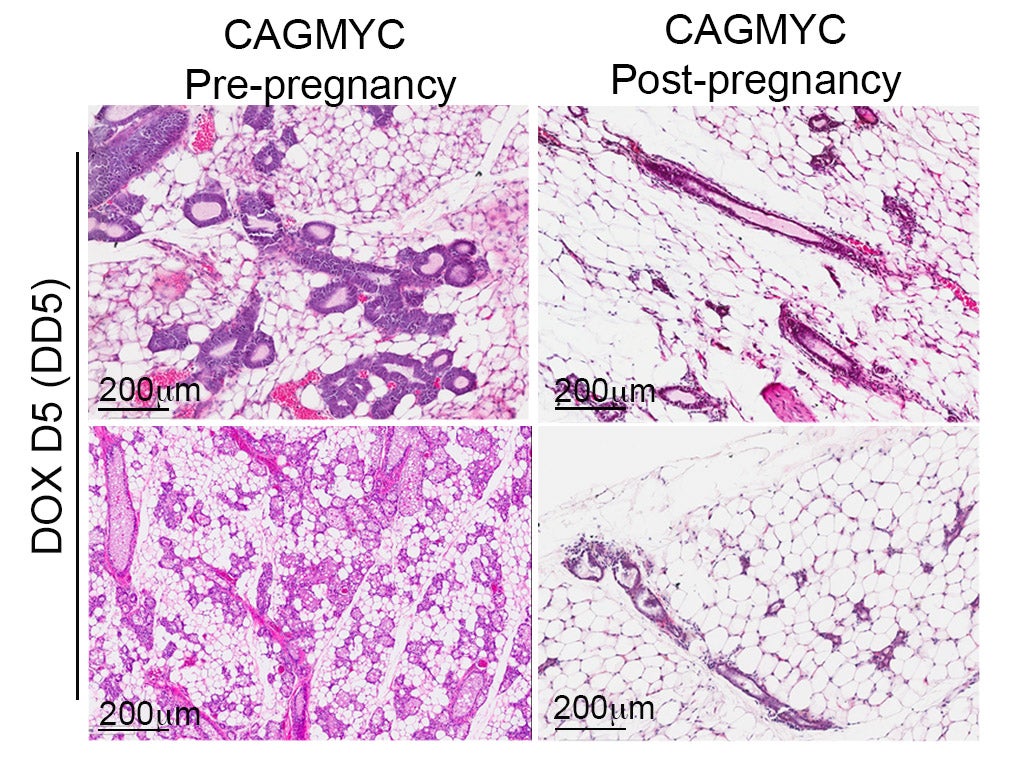
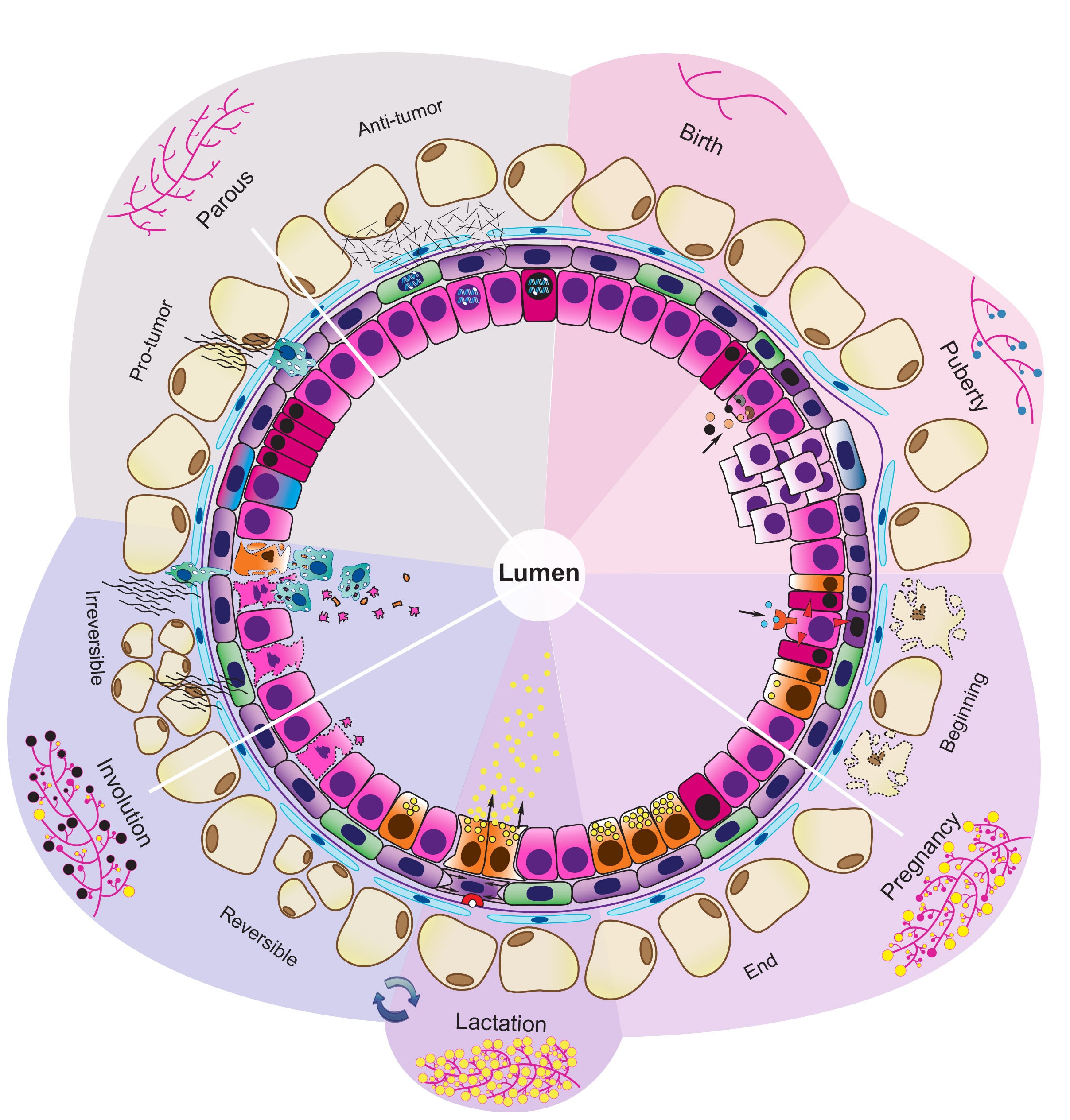
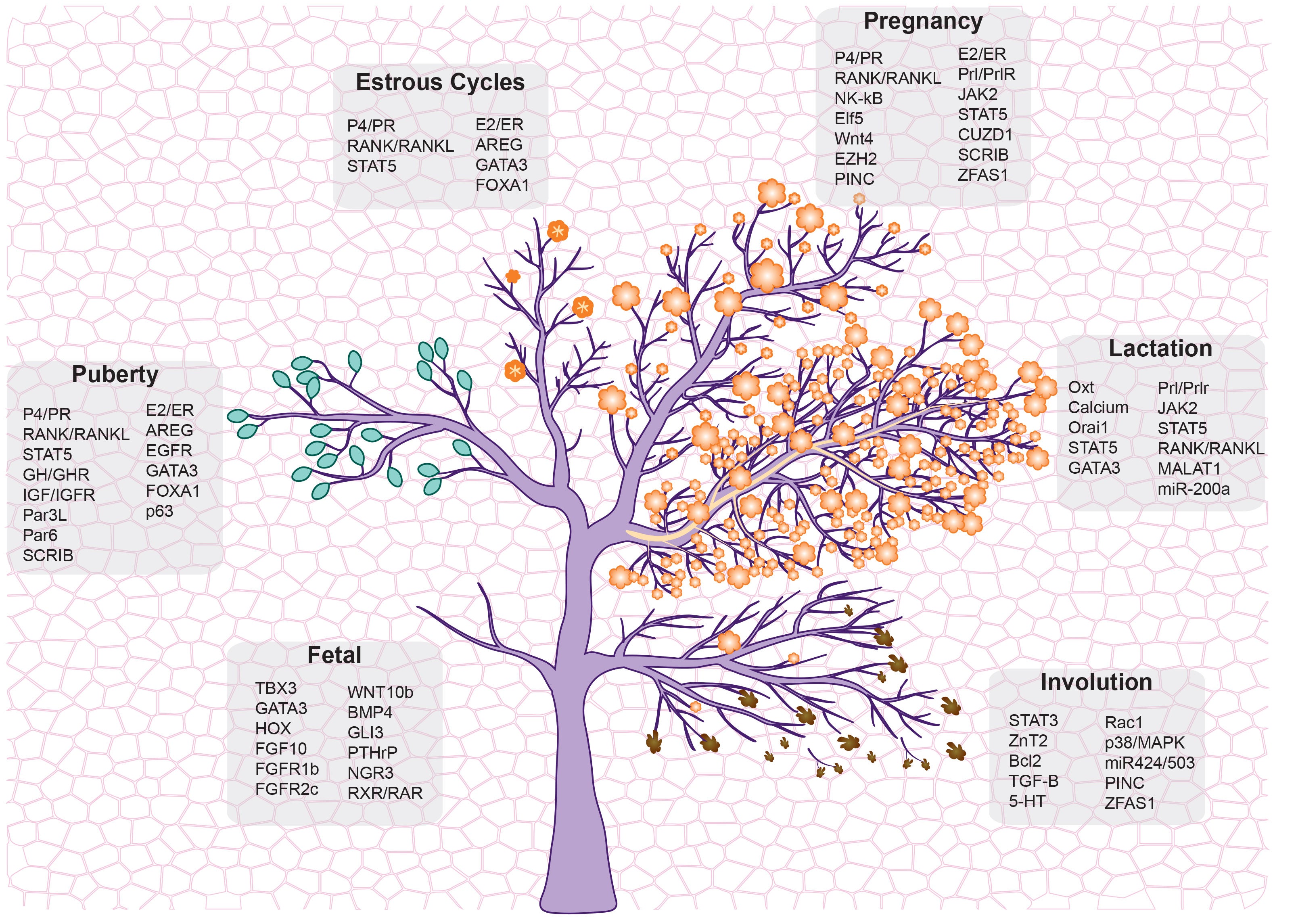
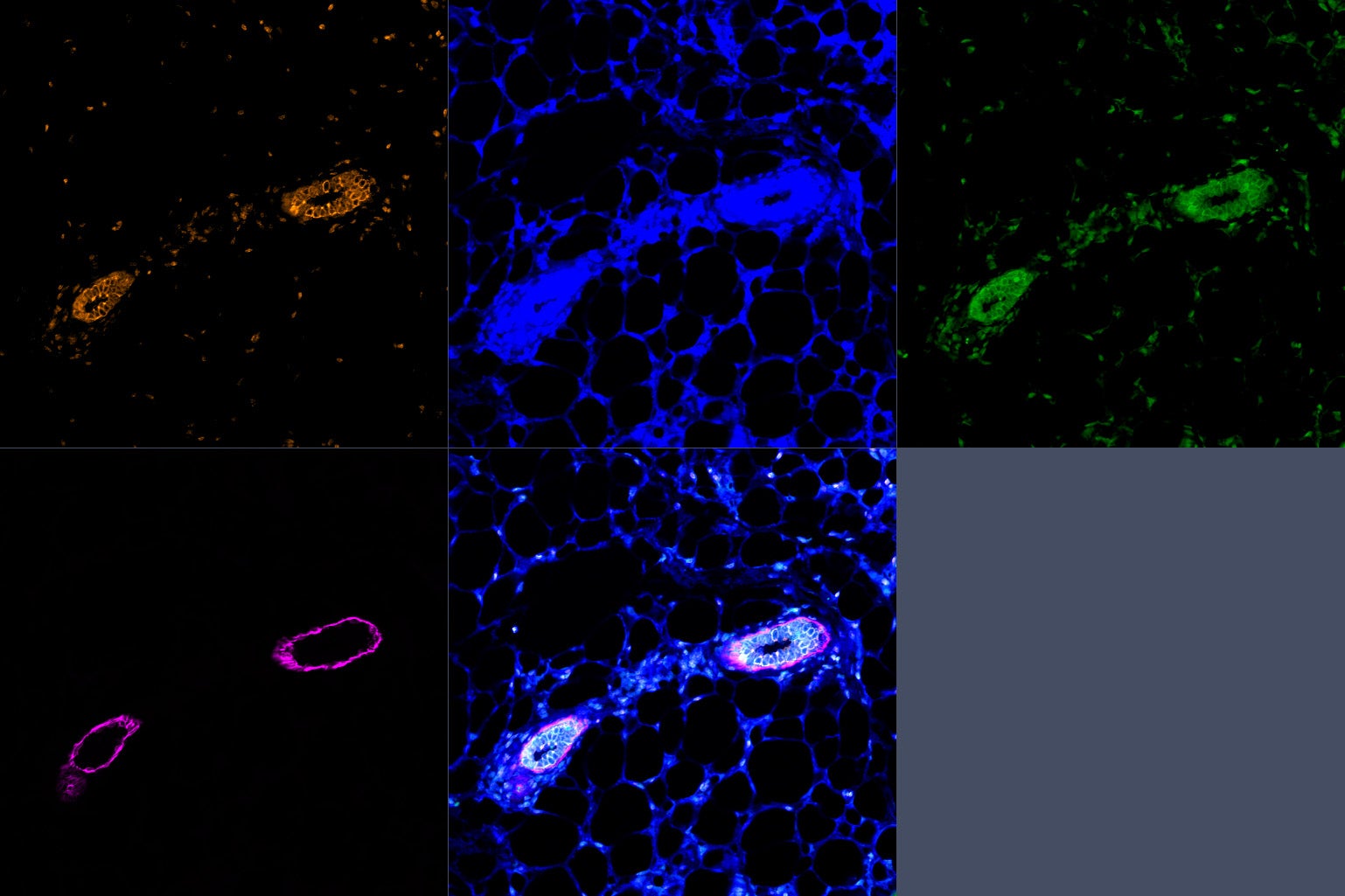
Cancer represents one of the most important challenges of modern medicine. One path to improve outcomes for breast cancer patients is develop next-generation ‘smart’ therapies for this disease, or alternatively develop strategies that prevent this disease altogether. For the last several years our laboratory has focused on identifying ‘epigenetic’ vulnerabilities in this disease, that is, approaches to alter how genes are expressed to prevent cancer development, and to attenuate the aggressiveness of breast cancer cells.
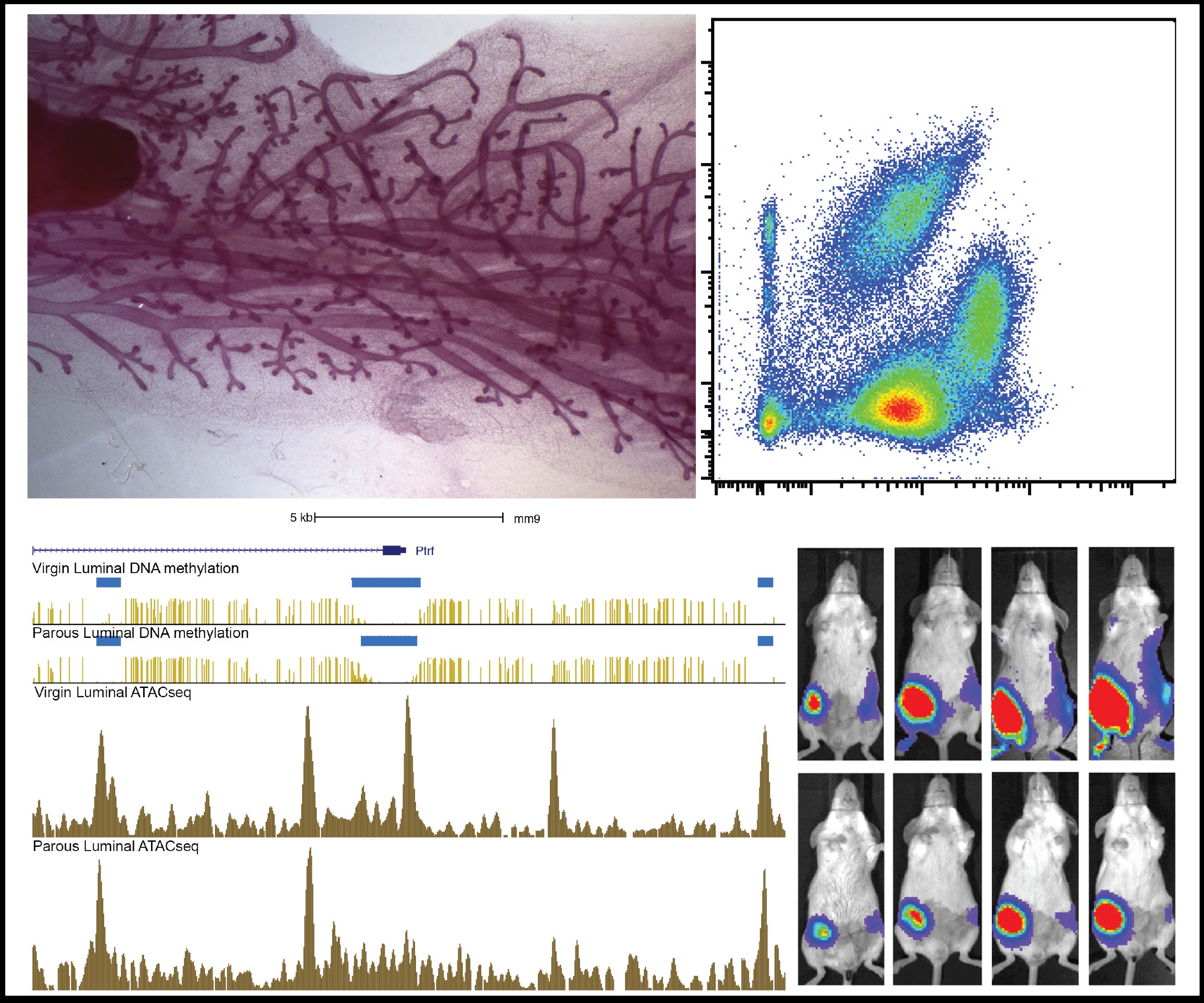
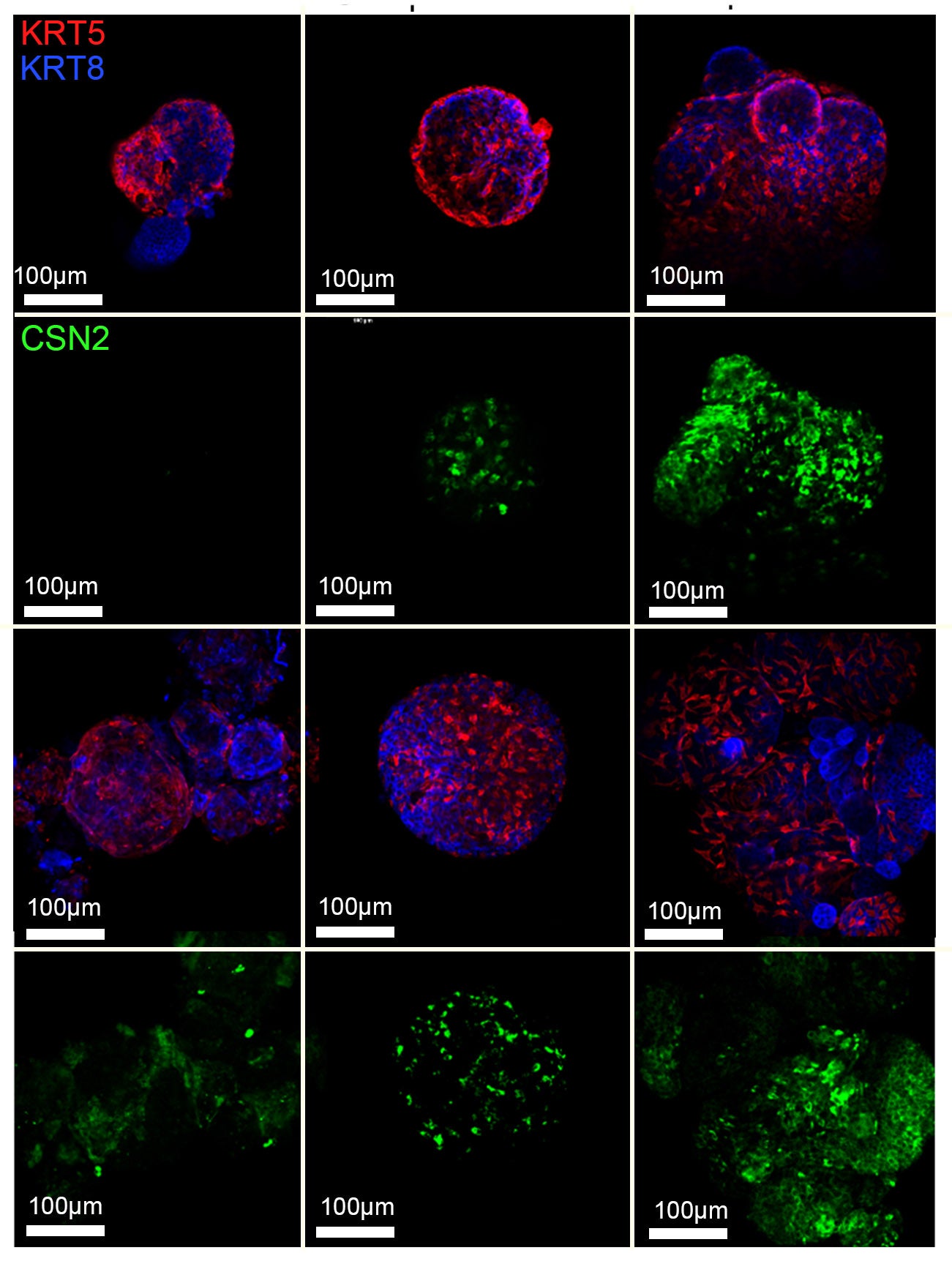
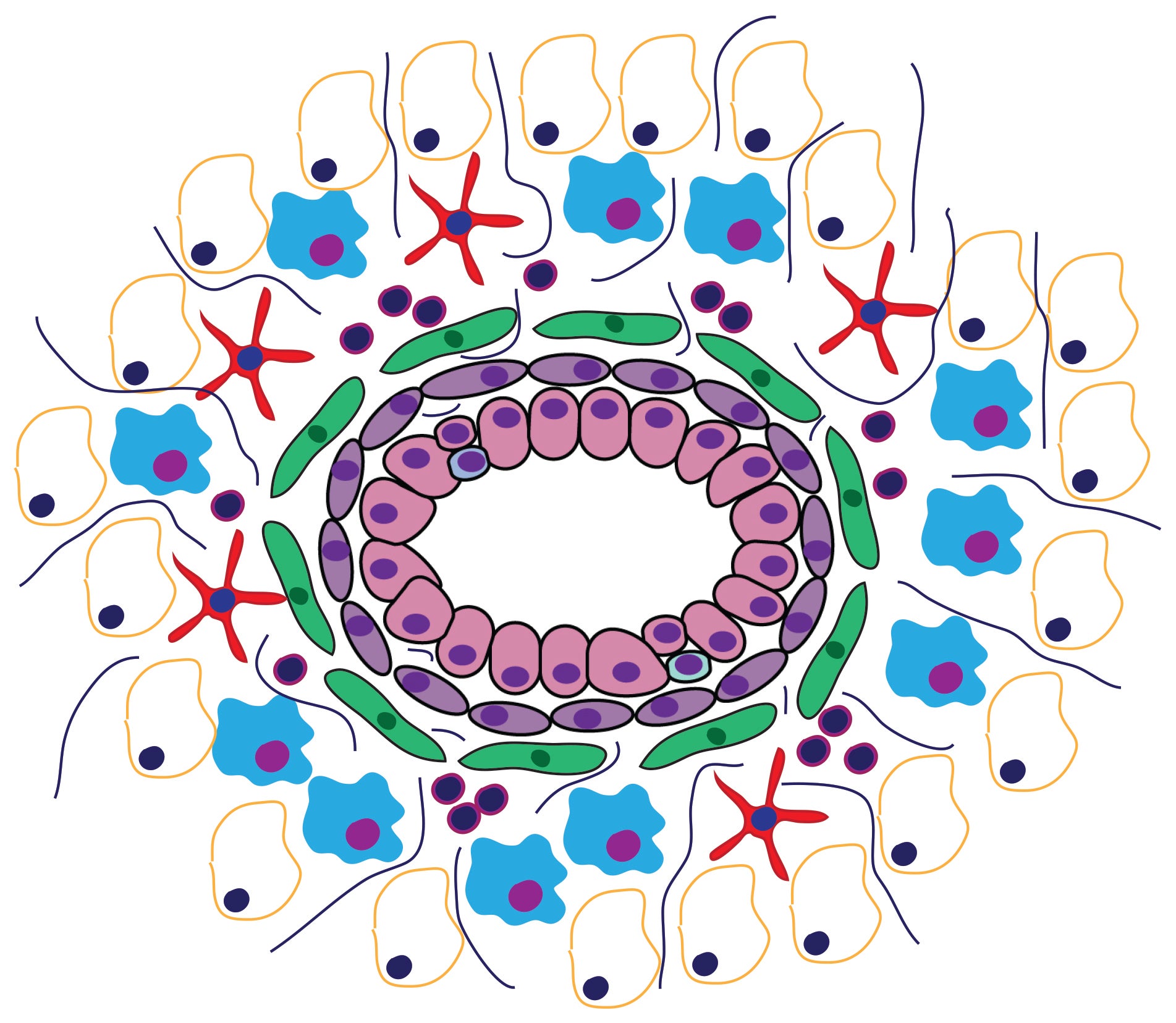
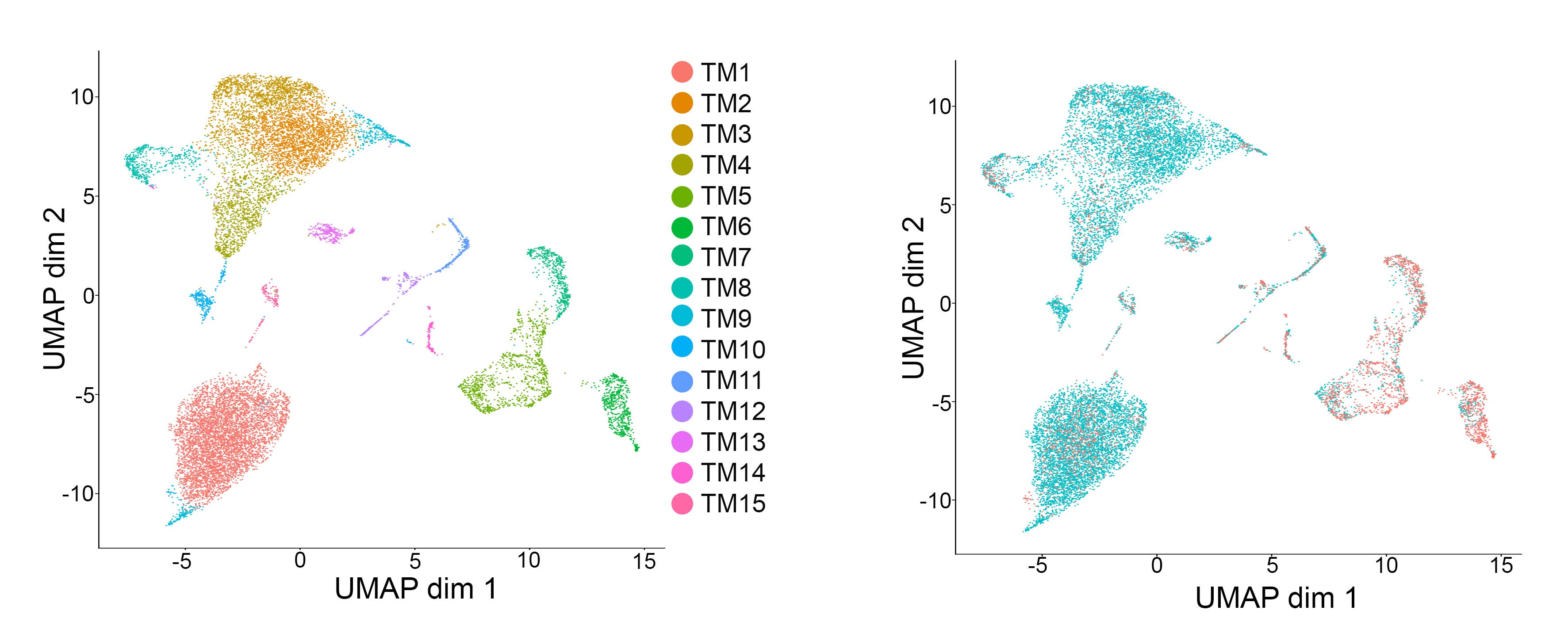
To date, we have developed a series of modeling systems (transgenic mouse, organoid systems) and analytical pipelines for epigenomics and transcriptomics analysis, which collectively provided evidence of cell autonomous and non-autonomous signals that play a role in blocking breast tumorigenesis.
Collectively, our studies aim to dissect the pathogenic mechanisms underlying breast cancer development and immune communication to inform the development of future diagnostic approaches to accurately predict and modulate cancer risk, and improved disease targeting.